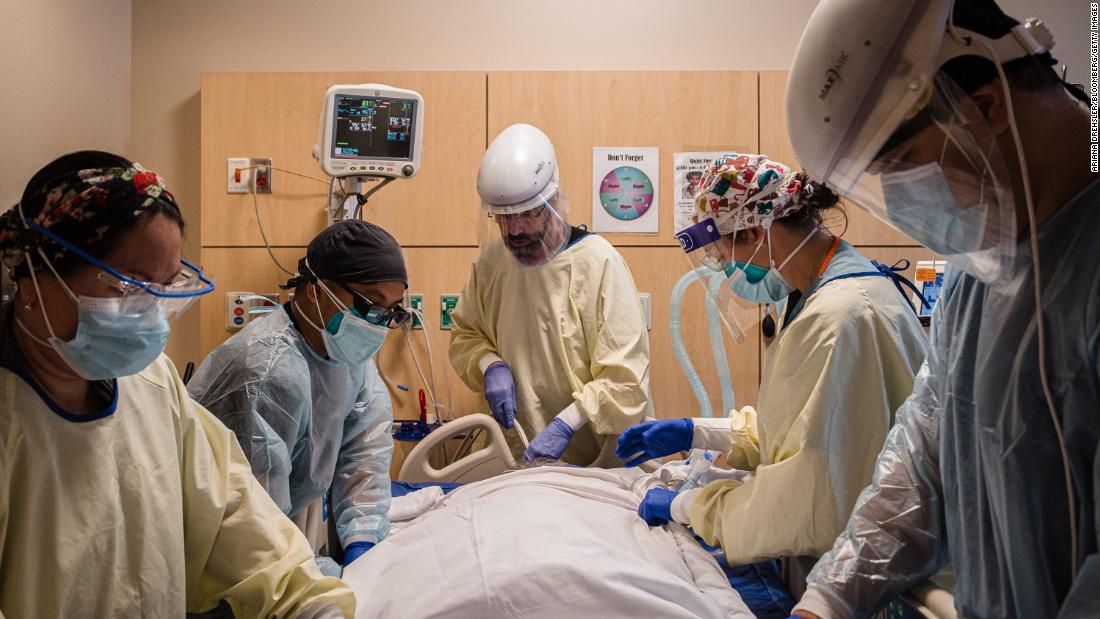
After weeks of tumbling case numbers, new infections are on the rise again
“Please hear me clearly: At this level of cases with variants spreading, we stand to completely lose the hard-earned ground we have gained,” said Dr. Rochelle Walensky, director of the US Centers for Disease Control and Prevention.
“Similarly, the most recent seven-day average of deaths has also increased more than 2% … to nearly 2,000 deaths per day.”
“I am really worried about reports that more states are rolling back the exact public health measures we have recommended to protect people from Covid-19,” she said.
“Please stay strong in your conviction. Continue wearing your well-fitting mask and taking the other public health prevention actions that we know work,” Walensky added.
“Ultimately, vaccination is what will bring us out of this pandemic. To get there, we need to vaccinate many more people.”
A 3rd vaccine will help with mass vaccination
The good news this week: Johnson & Johnson’s single-dose vaccine will start being administered.
“There’s a kind of new urgency to it,” said Dr. Eric Rubin, a professor at the Harvard T.H. Chan School of Public Health.
“Because there are new viral variants coming out right now, with some chance that some of them could eventually become somewhat resistant to the protection provided by vaccines, it’s really important to get it out there fast.”
About 3.9 million doses of the J&J vaccine will be distributed to states, tribes, territories, pharmacies and community health centers, a senior Biden administration official said Sunday night.
“Those J&J doses will be delivered as early as this Tuesday morning.”
As a one-dose vaccine, “people do not have to return for a second dose to be protected,” Walensky said.
“In addition, this vaccine does not need to be kept in a freezer and can be stored at refrigerated temperatures. So it is easy to transport and store and allows for expanded availability in most community settings and mobile sites as supply scales up.”
The other two vaccines being distributed — one from Pfizer-BioNTech and one from Moderna — both have efficacy rates of about 95%, with even greater protection against severe forms of disease.
The Johnson & Johnson vaccine has shown a 72% efficacy rate against mild to severe/critical disease among US trial participants. It’s more effective against severe forms of disease, with about 85% protection.
Health experts say Americans should not be deterred by J&J’s slightly lower numbers. Its vaccine was tested later than the other two vaccines, when infections were already surging and new variant strains were spreading more widely.
Don’t be too picky about which vaccine you get
“Think about the other vaccines that we get. If we go in to get the flu vaccine, I don’t think anyone is asking the brand of the flu vaccine (or) what company manufactures it.”
Besides, “in the immediate future, people are not going to be getting a choice when supply is a limiting factor,” she said.
“Right now, the key is to get immunity of some kind. Get whatever vaccine is first available to you. You can always get another vaccine or booster shot later on when supply is not the issue.”
New variants keep spreading
Those counts are probably much lower than the actual number of people infected by variants. The numbers represent only those variant cases found through genomic sequencing, the CDC has said.
Can vaccines fend off the new variants?
Johnson & Johnson, Moderna and Pfizer-BioNTech are trying to make sure their vaccines get ahead of the variants.
The B.1.351 strain first detected in South Africa has the most worrying effects on the ability of vaccines to produce an immune response, Dr. Heather Scobie said at the CDC’s Advisory Committee on Immunization Practices (ACIP) meeting on Monday.
She also said two doses of the Covid-19 vaccine protect people better than just one dose.
“Five studies have shown that postponing the second mRNA dose may leave some people less protected against the SARS-Cov-2 variants,” Scobie told the ACIP meeting.
The Moderna and Pfizer/BioNTech vaccines are mRNA vaccines.
“All of the studies showed improved neutralization of B.1.1.7 and B.1.351 after the second vaccine dose,” she said. “In a few studies, people who had recovered from Covid-19 and received one vaccine dose had moderate protection against B.1.351.”
Johnson & Johnson is working on a booster to help its Covid-19 vaccine deal with new strains of coronavirus variants, CEO Alex Gorsky said Monday.
“While we’re encouraged and we’re confident in the current vaccine that we have, you’ve always got to be preparing for the future and frankly for the unknown,” Gorsky said.
That shot would serve as a booster for people who have already been vaccinated and as a primary vaccine for people who haven’t had coronavirus and have not yet been vaccinated.
Moderna is also testing a third, lower dose of its current vaccine in trial participants to see if that would protect against troubling variants.
Pfizer and BioNTech said last week that they’ve started testing how well a third dose of their authorized vaccine stacks up against new variants.
On Monday, BioNTech CEO Ugur Sahin said it will take another six to eight weeks to get real-world data that shows how effective the Pfizer-BioNTech vaccine is against the B.1.351 variant.
Many more need vaccine before herd immunity
About 7.7% of Americans have been fully vaccinated with both doses.
Vaccines will be tested in children
“We will conduct several immunogenicity and safety studies in children from 17 years of age down to neonates,” Douoguih told a CDC advisory committee Sunday.
“The study in adolescents, we hope, will open next week. We are also anticipating a study in pregnant women in the second and third trimesters toward the end of March, early April,” Douoguih said.
Johnson & Johnson also plans to begin study in immunocompromised people in the third quarter of this year, Douoguih said.
The FDA’s emergency use authorization for the Johnson & Johnson vaccine is currently for use in adults 18 and older.
CNN’s Jen Christensen, Fred Pleitgen, John Bonifield, Jason Hoffman, Michael Nedelman and Maggie Fox contributed to this report.
![]()