The United Kingdom has become the first Western nation to begin vaccinating its citizens with a Covid-19 shot outside of clinical trials
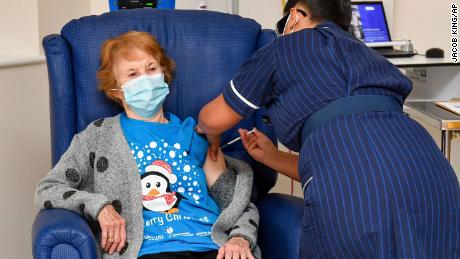
Keenan, who turns 91 next week, said she felt “privileged” to be first to get the shot.
“It’s the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the New Year after being on my own for most of the year,” she said, according to a statement released by the UK’s National Health Service (NHS).
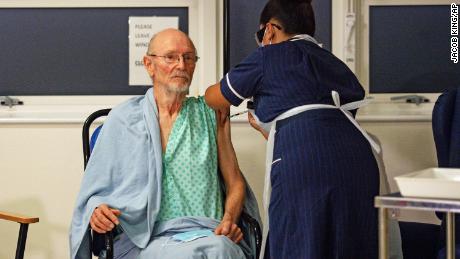
William Shakespere, 81, known to friends as Bill, was the second person to get a Covid-19 vaccine at the University Hospital in Coventry on Tuesday. Shakespeare, a patient on the hospital’s frailty ward, sat with his grandchildren’s artwork nearby as he received the jab.
In London, George Dyer become one of the first people in the capital to get the jab at Croydon Health Services while nurse Joanna Sloan was the first person in Northern Ireland to receive the vaccine.
May Parsons, the nurse who administered the first jab, said she was honored to be involved in the program. “The last few months have been tough for all of us working in the NHS, but now it feels like there is light at the end of the tunnel,” she said.
The UK has ordered 40 million doses of the vaccine, but only 800,000 shots will be available as part of the first wave that began on Tuesday.
Because the vaccine requires two doses, administered at least three weeks apart, the UK will have enough shots to vaccinate roughly a third of the country’s population. The country has also ordered 7 million doses of the Moderna vaccine, which could be approved for emergency use in the UK within the next few weeks.
There will be 50 vaccination hubs in hospitals across England and dozens more across Wales and Scotland. In England, the first wave of vaccinations will only be administered in hospitals. Wales and Scotland have yet to specify the types of locations they plan to have administer the vaccine.
UK Health Secretary Matt Hancock called the beginning of the vaccination program a “big moment in scientific endeavor” on Tuesday, saying that the vaccine “can start to heal this disease right across the world, and protect people everywhere.”
In an interview with Sky News on Tuesday morning, Hancock said there was still “an enormous amount of work to do,” but that it may be possible to lift the UK’s coronavirus restrictions in the spring if enough people have been vaccinated by then. He urged the public to “hold our nerve and stick together and follow the rules.”
While the UK’s immunization program started with a big bang on Tuesday, the vaccine remains off limits to the vast majority of people.
For now, it is available by invitation only for those age 80 and over, care homes staff and front line health and social care workers. Care home residents were also expected to be prioritized, but the government said last week that this won’t happen immediately.
The NHS said in a statement on Tuesday that vaccine centers would be set up in venues including sporting venues and conference centers to treat large numbers of patients once further supplies of vaccine become available.
Hari Shukla, 87, from Tyne and Wear in England’s north east, is due to be one of the first to get vaccinated at a hospital in Newcastle later on Tuesday. Ahead of the jab, he thanked the UK’s National Health Service for its work throughout the pandemic, saying that its staff “have a heart of gold.”
“I’m so pleased we are hopefully coming towards the end of this pandemic and I am delighted to be doing my bit by having the vaccine, I feel it is my duty to do so and do whatever I can to help,” Shukla said in a NHS statement.
The Pfizer/BioNTech vaccine offers 95% protection against Covid-19. According to the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), more than one in 10 recipients may suffer side effects including pain at the injection site, headache, muscle pain, chills, joint pain, and fever. A handful of other, less common, side effects are also listed.
The US Food and Drug Administration is expected to follow the UK’s MHRA and authorize the emergency use for the Pfizer vaccine as soon as this week — its vaccine advisory committee is scheduled to meet on Thursday.
The European Medicines Agency will conclude its decision on the vaccine on December 29, according to the European Commission.
CNN’s Nick Paton Walsh, Sharon Braithwaite and Kara Fox contributed to this report.
![]()